28+ Avogadro'S Number Calculator
Clear and comprehensive explanatory. Electron density distributions in space and energies eg.
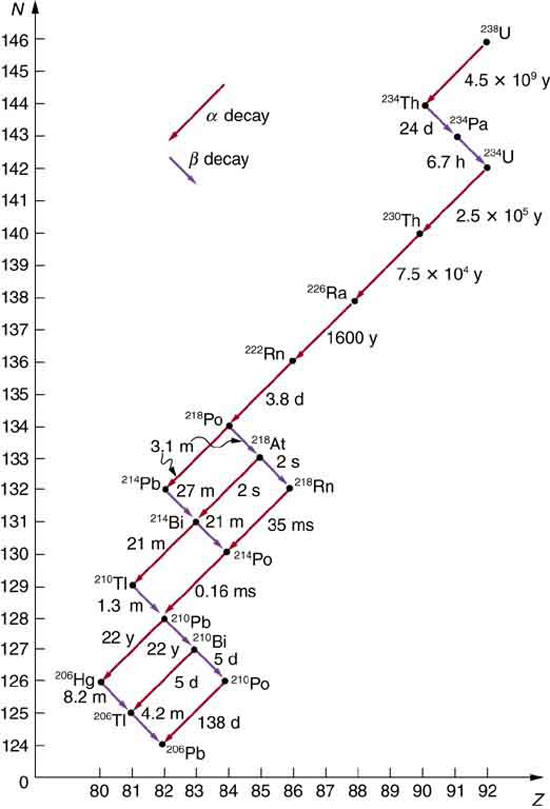
31 4 Nuclear Decay And Conservation Laws College Physics
Moles allow you to directly read weight from the periodic table eg 1 mole of N₂ is 28 g or 1 mole of NaCl is 585 g.
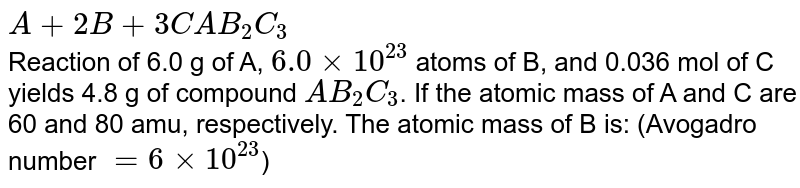
. Ibrahim Ismail October 9 2020 at 210 am. 123 Established and maintained by the General Conference on Weights and Measures. Your Mobile number and Email id will not be published.
Laws of Chemical Combination. N 2822400 x Volume of nitrogen at NTP Mass of compound x 100. 1s is lower energy than 2s which is lower energy than 3s.
Web There are a number of phenomena that explains various things which we observe in our everyday life. Mathematically density is defined as mass divided by volume. Web The International System of Units known by the international abbreviation SI in all languages.
Created by Sal Khan. Web Since the 2019 redefinition of SI base units both N A and k are defined with exact numerical values when expressed in SI units. Go Direct Radiation Monitor.
The carbon atomic weight is 12 units of atomic mass amu so the weight of one mole is 12 grams. A hydrogen atom is an atom with one proto. All answers are well explaned.
The first 94 elements of the periodic table are naturally occurring while the rest from 95 to 118 have only been synthesized in laboratories or nuclear reactors. Law of Mass Action. Though they sound similar they are known to occur completely in different terms.
The oxidation number of N 3-is -3. Therefore the formula of metal. Platinum-Cell Conductivity Probe Stainless Steel Temperature Probe.
S p d f and so on are the names given to the orbitals that hold the electrons in atoms. Leave a Comment Cancel reply. The atoms in He and N 2 for example have oxidation numbers of 0.
Web In case youre wondering Avogadros constant one of many constants stored in this calculator and available at the touch of a button used to be quoted as 6022045 10 23 since 2011 newer sources have given a more accurately calculated value of 6022141 10 23. Therefore 6022 x 10 23 electrons carries a charge of 6022 x 10 23 x 16023 x 10 -19 Cmol 96485 Cmol. Formula of the carbonate of a metal M is M 2 CO 3.
Although we sometimes miss focussing on these little things and these help us to keep life going on earth. Web This is because an additional number of neutrons compensates for the difference in the number of nucleons. View the biographies of math or Ask the Experts your questions on math.
The Base Hydrolysis of Ethyl Acetate. Go Direct Gas Pressure Sensor Go Direct Temperature Probe. Web The atomic and ionic radii of the transition elements decrease from group 3 to group 6 due to the poor shielding offered by the small number of d-electrons.
Some have suggested that it might be appropriate to name the symbol R the Regnault constant in honour of the. Law of Mass Action. Web Its equal to Avogadros number 602 X 1023 of atoms.
Avogadros numbers N 0 represents 6022 10 23 particles. Its important to be able to quantify what we can in a world of abstract. Web Avogadros Law.
Required fields are marked Send OTP. For example the oxidation number of Na is 1. Using the Avogadro number provides a convenient way of considering the weight of substance and the theoretical yield of chemical reactions.
Double bonds have a bond order of 2. The example of two Isotopes and Isobars is iron and nickel. And an oxygen atom is one with eight protons.
Required fields are marked Send OTP. Adhesion and cohesion forces are one of two phenomena. Web Nickel - Nickel is a chemical element with atomic number 28 and symbol Ni.
Both have the same mass number which is 58 whereas the atomic number of iron is 26 and the atomic number of nickel is 28. The number of electrons surrounding the nucleus determines whether an atom is electrically charged or neutral. Where ρ is the density m is the mass and V is the volume.
Your Mobile number and Email id will not be published. Gandhar telse December 28 2019 at 1219 pm. Its symbol is NA or L.
We can count the number of sheep on a farm or we can calculate the gallons of a cows milk. Know the nickel atomic number Chemical Properties of Nickel Atomic Mass and NiCl42 Geometry at BYJUS. Web The number of protons in an atoms nucleus distinguishes it from other atoms of the same element.
Those placed between groups 7 and 10 have somewhat similar atomic radii and those placed in groups 11 and 12 have larger radii. It can be found by drawing the Lewis structure of the molecule and counting the total number of electron pairs between the atoms in question. Web 1 mole of electrons is represented by the Avogadros Number L 6022 x 10 23 electrons.
Web The bond order of a covalent bond is the total number of covalently bonded electron pairs between two atoms in a molecule. Web Avogadros number represents how many particles. Web That number is known as Avogadros constant.
Laws of Chemical Combination. A carbon atom is an atom with six protons. 2s is lower energy than 2p.
Law of Constant Proportions. Iii and sometimes pleonastically as the SI system is the modern form. Web Density volumetric mass density or specific mass is the substances mass per unit of volumeThe symbol most often used for density is ρ the lower case Greek letter rho although the Latin letter D can also be used.
The oxidation number of a monatomic ion equals the charge of the ion. Single bonds have a bond order of 1. Web free flashcards for math students everywhere.
Web The n number determines how many of the subshells make up the shell. The usual oxidation number of hydrogen is 1. For each element the atomic weight is on the periodic table right under the symbol of the element.
Thanks for this valuable information. Web Precipitation Reaction is an chemical reaction occurring in aqueous solutions where two ionic bonds combine forming up insoluble salts. Web The number 6022 10²³ is known as Avogadros number or Avogadros constant.
Web Therefore as the energy level of the atom increases the number of energy sub-levels per energy level increases. Tanishq May 19 2020 at 1128 pm. 117 of the metric system and the worlds most widely used system of measurement.
The valency of the metal M in M 2 CO 3 is 1 ie metal exists as M ion. As a consequence the SI value of the molar gas constant is exactly 8314 462 618 153 24 JK 1 mol 1. Law of Constant Proportions.
Web The oxidation number of a free element is always 0. These orbitals have different shapes eg. Exploring the Properties of Gases.
Write the formula of its chloride. Go Direct Constant. The concept of the mole can be used to convert between mass and number of particles.
Web Avogadros Law. Learn addition subtraction multiplication and division with our free easy to use arithmetic flash cards. Best for Kids 12 and under.
Learn more about the definition examples equations of precipitation reaction.

Kinetic Energy Examples Pdf Examples

Calculating Moles Using Avogadro S Number Youtube

1st Puc Chemistry Question Bank Chapter 5 States Of Matter Kseeb Solutions
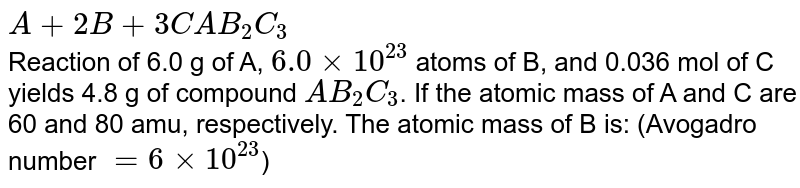
A 2b 3c Ab2 C3 Reaction Of 6 0 G Of A 6 0 Xx 10 23 Atoms Of B And 0 036 Mol Of C Yields 4 8 G Of Compound Ab 2 C 3 If The Atomic
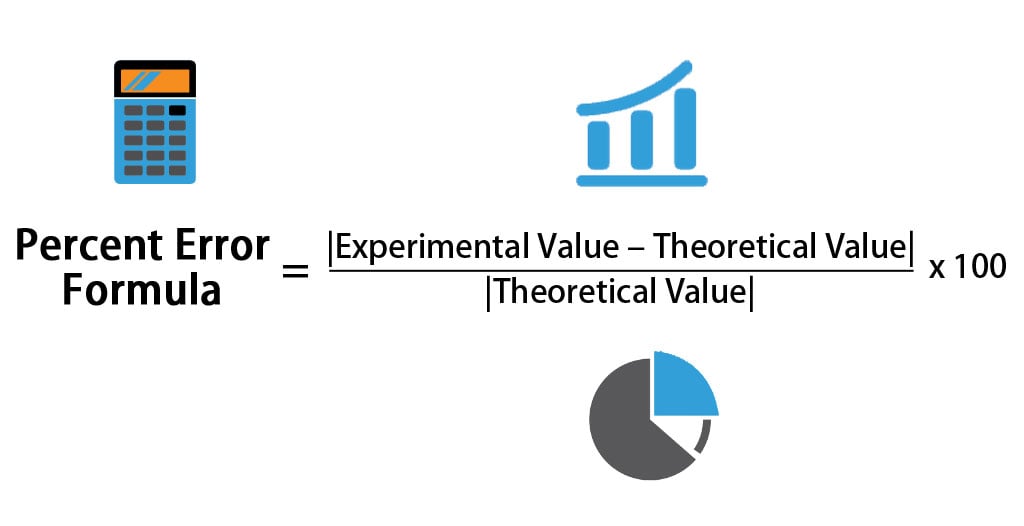
Percent Error Formula Calculator Excel Template

Avogadro S Number Calculator

A 2b 3c Ab2c3 Reaction Of 6 0 G Of A 6 0 10 23 Atoms Of B And 0 036 Mol Of C Yields 4 8 G Of Compound Ab2c3 If

Avogadro S Law Calculator Thermodynamics Heat Online Unit Converters

Avogadro S Number Chemistry Quiz Quizizz

Notes On Essential Chemistry Eb 3237 Essential Chemistry Nilai Thinkswap
Calculator Help Griger Science
Full Article Hydrogenic Systems Frequency Standards And Fundamental Constants
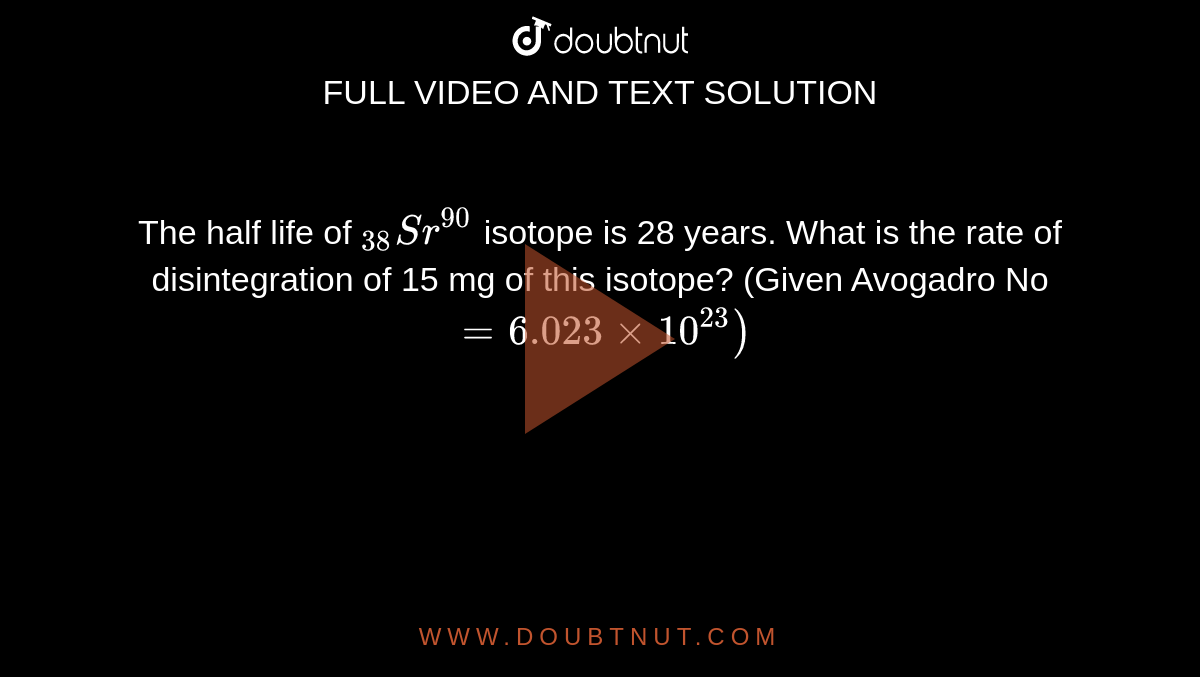
The Half Life Of 38 Sr 90 Isotope Is 28 Years What Is The Rate Of Disintegration Of 15 Mg Of This Isotope Given Avogadro No 6 023 Xx 10 23

Physical Chemistry 1 Relative Mass The Mole And Avogadro S Constant Slides Student Led Tasks Teaching Resources
Properties Avogadro Constant Calculator Org

1st Puc Chemistry Question Bank Chapter 5 States Of Matter Kseeb Solutions

Avogadro S Number The Mole Grams Atoms Molar Mass Calculations Introduction Youtube